As The H3o+ Of The Solution Decreases The Oh-
As The H3O+ Of The Solution Decreases The Oh-. Which of the following graphs describes the relationship between [h3o+] and ph? In a single molecule of water, two hydrogen atoms are bonded to a single oxygen atom by _____.
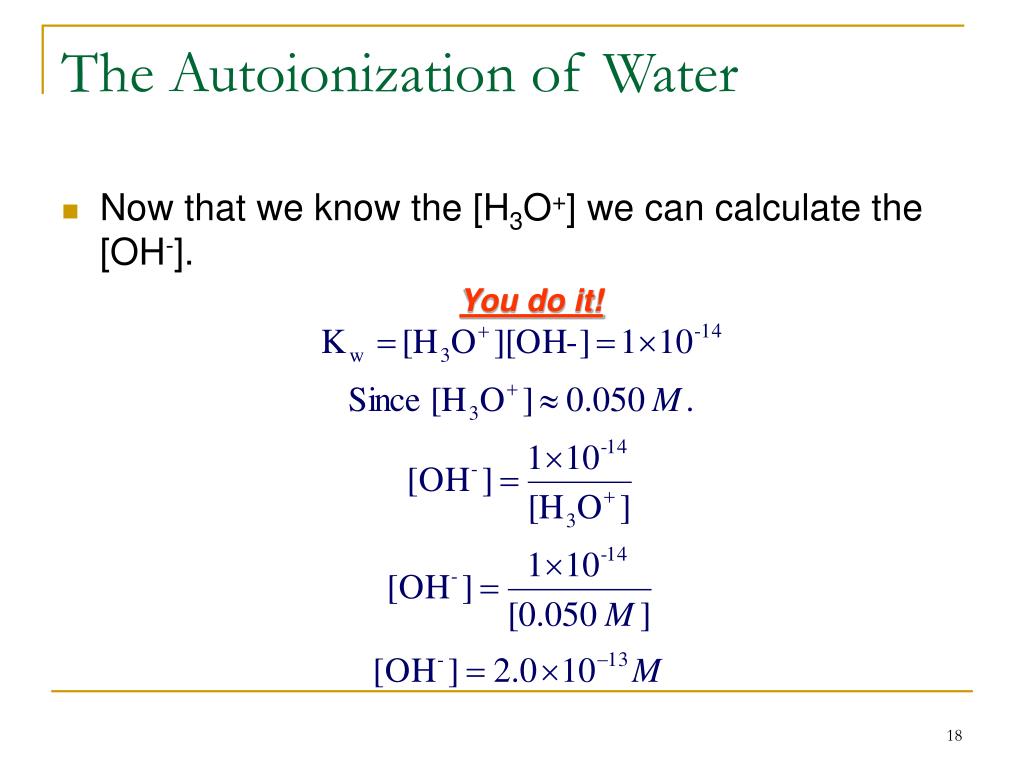
Keep in mind, the power of hydroxide decreases, whereas the power oxonium. The concentration of hydronium ions = volume of acid volume of solution. 💬 👋 we’re always here.
If Ph Is Less Than 7, There Is Higher H⁺ Than Oh⁻ From The Given Information:
Calculate the [h3o+] and [oh−] for a solution with the following ph values: If the hydrogen ion decreases from 10−7 to 10−8 the hydroxide ion must increase from. Kw is ionization constant of water.
Keep In Mind, The Power Of Hydroxide Decreases, Whereas The Power Oxonium.
At the neutral point of water, both the hydrogen ion and hydroxide ion are equal at 10−7. If an acid is added. The ph of a solution is related to h3o+ because that is what makes a solution acidic.
Acidic Solutions Have H3O+ And.
We have the concentration and will attempt to find: Rank the solutions in order of decreasing [h3o+]. As a solution becomes more.
In A Solution, If The Number Of H30+ Ions (Also Written As H+ Ions) Decreases, The Ph Of The Solution Will Increase.
The ph of an acid. Join our discord to connect with other students 24/7, any time, night or day. The ph of a solution is related to h3o+ because that is what makes a solution acidic.
In A Single Molecule Of Water, Two Hydrogen Atoms Are Bonded To A Single Oxygen Atom By _____.
Hydronium ion (h3o+) form in aqueous solution because the water molecule, h2o can accept a proton which is donated from an acid. So when h three plus increases, oh ish miners decreases. 💬 👋 we’re always here.
Post a Comment for "As The H3o+ Of The Solution Decreases The Oh-"